What Are Dna Repair Enzymes
The DNA Repair Pathways
A variety of endogenous and exogenous DNA-dissentious agents such equally UV light, ionizing radiation (IR) and chemotherapeutic agents tin can atomic number 82 to Dna lesions, including mismatches, single-strand breaks (SSBs), double-strand breaks (DSBs), chemic modifications of the bases or sugars, and interstrand or intrastrand cantankerous-links. If the damage is non corrected, information technology will cause genomic instability and mutation, which is one of the cancer hallmarks (Hanahan and Weinberg, 2022). In order to forbid this situation, cells have evolved a series of mechanisms called DNA damage response (DDR) in society to deal with such lesions. DDR is a complex network that functions in unlike means to target various Dna lesions, including point transduction, transcriptional regulation, cell-bike checkpoints, induction of apoptosis, impairment tolerance processes, and multiple DNA repair pathways (Figure ane) (Giglia-Mari et al., 2022; Tian et al., 2022).
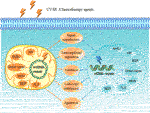
FIGURE one. Dna harm response. Deoxyribonucleic acid impairment is acquired past endogenous agent oxygen species (ROS) or exogenous agents such as UV low-cal, ionizing radiation (IR) and chemotherapy agents. Deoxyribonucleic acid damage response (DDR) is induced to deal with the lesions, including point transduction, transcriptional regulation, cell-bicycle checkpoints, induction of apoptosis, multiple Deoxyribonucleic acid repair pathways as well equally harm tolerance processes. DNA repair pathways include nuclear and mitochondrial DNA repair pathways. Direct repair, BER, MMR and recombinational repair (HR and NHEJ) are existence in both nuclear and mitochondrial repair systems. NER has been reported just appearance in nucleus, and the being of TLS pathway in mitochondria is unknown. NDNA, nuclear Deoxyribonucleic acid; MtDNA, mitochondrial DNA; BER, base excision repair; HR, homologous recombination repair; NHEJ, not-homologous end joining; MMR, mismatch repair; TLS, translesion synthesis; NER, nucleotide excision repair.
In mammalian cells, the ii chief organelles containing DNA are nucleus and mitochondria. Nuclear Deoxyribonucleic acid (nDNA) repair systems are divided into the following major pathways: 1) straight reversal, which mainly repairs the lesion induced by alkylating agents, ii) base excision repair (BER), aiming at Dna breaks (SSBs) and non-bulky dumb DNA bases, 3) nucleotide excision repair (NER), correcting bulky, helix-distorting Deoxyribonucleic acid lesions, 4) mismatch repair (MMR), repair of insertion/deletion loops (IDLs) and base-base mismatch, 5) recombinational repair, which is further divided into homologous recombination repair (HRR) and not-homologous end joining (NHEJ), primarily functioning at Deoxyribonucleic acid double strand breaks, half dozen) alternative nonhomologous terminate joining (alt-NHEJ, MMEJ), involved in repair of DSBs, 7) translesion synthesis (TLS), which is more likely to be a DNA damage tolerance mechanism (Jackson and Bartek, 2009; Hosoya and Miyagawa, 2022). Mitochondrial Dna (mtDNA) repair pathways, including the directly reversal, BER, MMR, TLS and double-strand break repair (DSBR), can repair damaged DNA to maintain mitochondria genetic integrity, protect mtDNA confronting oxidative harm, and promote cell survival (Ohta, 2006; Saki and Prakash, 2022).
Role of DNA Repair Pathways in Cancer Biology
DNA repair pathways play an of import role in the maintenance of genome stability and integrity through correcting the impaired Deoxyribonucleic acid that may contribute to carcinogenesis (Clementi et al., 2022). Numerous studies accept indicated that certain cancers are associated with the defect or mutation in the proteins of nuclear or mitochondrial Dna repair pathways (Pearl et al., 2022; Cerrato et al., 2022). For example, the defect in the ATM–Chk2–p53 pathway, which plays a crucial role in DNA double-strand breaks repair, promoted glioblastoma multiforme (GBM) germination and contributed to GBMs radiation resistance (Squatrito et al., 2010). The homo syndrome hereditary nonpolyposis colorectal cancer (HNPCC), which connects with high degrees of microsatellite instability, is caused by germline mutations in MMR genes, and the tumorigenesis of this affliction is connected with the defect in the MMR pathway (Hampel et al., 2005). People who deport an MMR cistron mutation accept the increased hazard of a wide variety of cancers than their noncarrier relatives (Win et al., 2022). Two important homologous recombination (60 minutes) Dna repair-related genes, BRCA1 and BRCA2 germline mutant confer the genetic predisposition to breast, ovarian cancer and pancreatic cancer (Riaz et al., 2022). In improver, the tumor microenvironment characteristic of hypoxia, low pH and nutrient deficiency, can give rising to genomic instability and tumor progress through downregulating DNA repair pathway. It has been reported that hypoxic circumstance can result in the reduction of MLH1 expression, a core protein in the MMR pathway (Mihaylova et al., 2003). The downregulation of RAD51, a key mediator of HRR, was observed in multiple cancer cell types induced by hypoxia, suggesting that the hypoxic tumor microenvironment can suppress the HRR pathway to cause genetic instability (Bindra et al., 2004; Lu et al., 2022). Tumor hypoxia besides regulated the DDR by driving alternative splicing (Memon et al., 2022). Report in human pulmonary epithelial cells has found that the acidic conditions delayed DNA damaging compounds benzo[a]pyrene (B[a]P) metabolism and inhibited NER capacity, ultimately enhanced B[a]P-induced Dna harm (Shi et al., 2022). Recent studies have shown that extracellular nutrients accept pregnant effects on genome integrity. Glutamine is the main source of carbon and nitrogen for tumor cells. Lack of glutamine led to Dna alkylation harm by inhibiting ALKBH action and increased the sensitivity of cancer cells to alkylating agents (Tran et al., 2022). Glucose starvation also enhanced radiosensitivity of tumor cells by reducing Deoxyribonucleic acid double-strand intermission (DSB) repair (Ampferl et al., 2022). Thus, the dysregulation of DNA repair pathways can contribute to the evolution of cancer past promoting genomic instability and mutation in mammal cells.
Targeting DNA Repair Pathways in Cancer Therapy
The near mutual cancer treatments, including chemo- or radiotherapy, are designed to induce cell death by direct or indirect DNA damage. Notwithstanding, tumor cells can initiate DNA repair pathways to resist these anticancer agents during chemo- or radiotherapy. Therefore, combination of the nuclear or mitochondrial DNA repair pathway inhibitors with anticancer agents may increase the tumor prison cell sensitivity to these agents.
O-6-Methylguanine-DNA Methyltransferase (MGMT)
The office of MGMT is to remove alkyl adducts from the Ohalf dozen position of guanine. Thus, the protective effect of MGMT could diminish the cytotoxic effects of alkylating agents (Middleton and Margison, 2003), suggesting that MGMT activity is likely to be a useful marker of the sensitivity of cancer cells to alkylating agents. It has been reported that loftier MGMT expression in tumor jail cell is associated with the resistance to 1,three- bis- (2-chloroethyl) -i- nitrosourea (BCNU) and temozolomide (TMZ) (Happold et al., 2022; Hsu et al., 2022), which target the Osix-position of guanine, resulting in cytotoxic and mutagenic DNA adducts (Rabik et al., 2006). Recently, researchers establish that MGMT-mediated the resistance to DNA alkylating agents in cancer cell is profoundly dependent on the Dna repair enzyme PARP. Combination of temozolomide with PARP inhibitors (PARPi) in MGMT-positive cancer cells enhanced the anticancer effects (Erice et al., 2022; Jue et al., 2022).
The inactivation of MGMT in tumor cells has been appreciated as a therapeutic target for sensitizing cells to Ohalf-dozen-alkylating agents (Maki et al., 2005). In vitro and in vivo studies demonstrated that Ohalf dozen-Benzylguanine (O6-BG), a typical pseudo-substrate that was developed to inactivate MGMT, in combination with O6-alkylating agents increased the therapeutic efficacy of chemotherapeutic alkylating agents (Maki, Murakami, 2005). Lomeguatrib (called Ovi-(4-bromothenyl) guanine, besides as PaTrin-2), another pseudo-substrate tested in clinical trials, has been shown to increase the therapeutic alphabetize of methylating agent temozolomide in nude mice bearing A375M human melanoma xenografts and patients with avant-garde solid tumors (Middleton et al., 2002; Ranson et al., 2006). Bobustuc GC et al. demonstrated that inhibition of MGMT suppressed the expression of survivin and enhanced the cytotoxicity of gemcitabine in pancreatic cancer (Bobustuc et al., 2022). Another approach to MGMT inactivation is to silence the MGMT cistron expression through its promoter methylation. Several studies in fauna models take suggested that the therapy of MGMT gene silence was able to overcome TMZ resistance and increment tumor cell death (Viel et al., 2022). Clinical report indicated that patients with glioblastoma containing a methylated MGMT promoter obtained more benefits from TMZ than those who did not accept a methylated MGMT promoter (Hegi et al., 2005). Lately, it has been confirmed that MGMT factor methylation tin can be a biomarker for temozolomide (TMZ) treatment and a potent prognostic factor in patients with GBM (Kim et al., 2022; Iaccarino et al., 2022; Zhao et al., 2022; Binabaj et al., 2022). However, according to the data from National Cancer database (NCDB) indicated that but 4.9% of GBM patients have MGMT promoter methylation. Even though MGMT promoter methylation status has prognostic value, it is ignored in the United States (Lee et al., 2022). More researches demand to conduct to place the prognostic value of MGMT promoter methylation in tumor patients responding to alkylating agents.
Base Excision Repair
A number of investigations take shown that inhibition of BER pathway can enhance the sensitivity of cancer cells to alkylating agents and radiotherapy (Neijenhuis et al., 2005; Gao et al., 2022). The principal methods to forestall the activity of BER pathway focus on the development of AP endonuclease 1 (APE1) or Poly (ADP-ribose) polymerase (PARP) inhibitors.
Several studies indicated that methoxyamine (MX), a small alkoxyamine that can bind with the costless aldehyde of AP site to forbid APE1 cleavage at AP sites, thereby inhibiting APE-1 endonuclease activity. Combined treatment with chemotherapeutic alkylating agent such as TMZ and BCNU could reinforce the cytotoxicity of alkylating amanuensis by targeting BER pathway (Liu et al., 2003; Montaldi and Sakamoto-Hojo, 2022). Recently, based on preclinical studies, several clinical trials were conducted, for example combination therapy with MX and TMZ in patients with advanced solid tumors has completed (NCT00892385). Currently, phase Ⅰ clinical trials of MX in combination of TMZ is undergoing in patients with relapsed solid tumors and lymphomas (NCT01851369). MX combination with pemetrexed disodium, cisplatin, is at present investigating in phase Ⅰ/Two phase in patients with advanced malignant solid neoplasm (NCT02535312). Lucanthone, a topoisomerase II inhibitor as well as an APE1 endonuclease inhibitor, has been shown to reinforce the cell killing effect of alkylating agents in human breast cancer cell line MDA-MB-231 (Luo and Kelley, 2004). Lucanthone combination with radiation and TMZ in GBM patients was tested in phase Ⅱ clinical trial (NCT01587144). Notwithstanding, it was terminated in 2022. Another stage II clinical trial investigating lucanthone combination with radiation in patients with brain metastases from non-modest cell lung cancer was withdrawn due to drug issues (NCT02014545).
PARP family unit is composed of 17 members, of which PARP1 and PARP2 are well-recognized Deoxyribonucleic acid damage sensors, particularly PARP1. PARP1 detect the region of damaged Dna and play a key role in several Dna repair pathway including BER, HHR and MMEJ (Konecny and Kristeleit, 2022). While PARP1 is best studied in BER and the mechanism of PARP inhibitor (PARPi) is based on trapping PARP1 on SSBs DNA site to inhibit BER repair. Finally, information technology converted SSBs into DSBs and impelled cell expiry in Hour-deficiency tumor, for case BRCA1/2 mutations, RAD51 deficiency (Figure ii) (Konecny and Kristeleit, 2022; Brown et al., 2022; Lord and Ashworth, 2022; Oplustil O'Connor et al., 2022). In 2005, two pre-clinical researches published in nature indicated that BRCA1 or BRCA2 deficient cells highly sensitized to PARP inhibition (Farmer et al., 2005; Bryant et al., 2005). Based on the concept of "synthetic lethality"-targeting either cistron solitary in a synthetic lethal pair is tolerated, simply simultaneous targeting both genes is lethal, researchers applied PARPi to BRCA mutation tumors (Dhillon et al., 2022). Several clinical trials using PARPi including Olaparib, Veliparib, Rucaparib (Table 1) as monotherapy for the treatment of patients with germline BRCA1/two mutation tumors including advanced chest cancer, ovarian cancer, pancreatic cancer and prostate cancer presented significantly antitumor consequence (Kaufman et al., 2022; Robson et al., 2022; Moore et al., 2022; Golan et al., 2022). Olaparib as maintenance therapy also significantly prolonged progression-free survival in advanced ovarian cancer patients with HRD-positive tumors who have achieved first-line standard therapy including bevacizumab. It has been approved by FDA for utilization of Olaparib in patients with advanced germline BRCA-mutated ovarian cancer following three or more prior lines of chemotherapy (Kim et al., 2022). On May 19, 2022, the FDA likewise canonical Olaparib for patients with metastatic castration-resistant prostate cancer (mCRPC) conveying HRR gene-mutated based on NCT02987543. PAPR1 inhibitors in combination with IR or with other unlike anticancer agents are currently undergoing clinical trials for treatment of patients with BRCA1/two mutation or HRR-deficiency advanced solid tumors, which shown promising clinical activity (Blindside et al., 2022; Wilson et al., 2022; Loibl et al., 2022; Coleman et al., 2022; Farago et al., 2022; Konstantinopoulos et al., 2022; Liu et al., 2022).
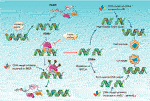
FIGURE 2. Mechanism and function of PARP and PARP inhibitors. The catalytic function of PARP1 is activated through bounden to the SSBs site cuased by alkylating agents. Activated PARP1 undergo PARylation and recruitment of a serials of fundamental Deoxyribonucleic acid repair effectors involved in BER to repair DNA lesion. Finally, PARP1 release from DNA and regain inactive state. PARP inhibitors binds the catalytic site of PARP and impaired of the enzymatic activity of PARP which "trap" PARP1 on DNA, results in suppression of the catalytic bicycle of PARP1 and BER. Trapping PARP1 on DNA lesion also collapses DNA replication fork, therefore transforming SSBs into genotoxic DSBs. This type of Deoxyribonucleic acid lesion would commonly induce HR for repairing damaged Deoxyribonucleic acid. Notwithstanding, if 60 minutes-lacking exist in tumor cells, including BRCA1/2 deficiency or mutation, some other less constructive and error-prone DSBs repair pathway NHEJ or alt-NHEJ could be utilized, which causing genomic instability, chromosomal fusions/translocations and afterward inducing cell death. SSBs, unmarried-strand breaks; DSB, double-strand break; BER, base excision repair; alt-NHEJ, alternative nonhomologous end joining; NHEJ, non-homologous end joining; HR, homologous recombination repair.
TABLE i. DNA repair pathway inhibitors in electric current clinical trials.
Double Strand Breaks Repair
Among various DNA lesions, DSBs is the leading lethal damage that leads to cell decease and genetic mutations. DNA-dependent poly peptide kinase (DNA-PK), a member of the PI3K-related poly peptide kinase (PIKK) family unit, is involved in DSBs repair pathway via not-homologous finish joining (NHEJ) (Jette and Lees-Miller, 2022). It has been reported that Deoxyribonucleic acid-PK activity plays a role in chemo-radiotherapy resistance (Wang Y. et al., 2022; Stefanski et al., 2022; Alikarami et al., 2022; Liu et al., 2022). Selective Deoxyribonucleic acid-PK inhibitor take been adult, including NU7026 (Dolman et al., 2022), NU7441 (Yang et al., 2022), IC87361 and SU11752 (Shinohara et al., 2005). They could inhibit DSBs repair pathway and raise the sensitivity of cancer cells to ionizing radiation or/and chemo-potentiation such as doxorubicin (Ciszewski et al., 2022). The combination of Dna-PK inhibitor M3814 with type Two topoisomerase inhibitors, including doxorubicin, etoposide and pegylated liposomal doxorubicin, enhanced the efficacy of type Two topoisomerase inhibitors in ovarian cancer xenografts (Wise et al., 2022). Several novel Deoxyribonucleic acid-PK inhibitors including MSC2490484A, VX-984 (M9831), M3814 are nether clinical trial as single-agent or combination with Chemo-radiotherapy (Tabular array 2). Alexander One thousand. Tsai et al. recently found that NU7441 combination with a multikinase inhibitor regorafenib altered immune microenvironment of melanomas and enhanced the efficacy of various immunotherapies (Tsai et al., 2022).

TABLE 2. Inhibitors of DNA repair pathway recently under preclinical studies.
Ataxia-teleangectasia mutated (ATM) and ATM-RAD3-related (ATR) protein, similar DNA-PK protein, are the members of PIKK family. They work as a transducer of the DSB betoken, and are involved in the repair of DNA DSBs (Weber and Ryan, 2022). A large of ATM inhibitors, including KU-55933, KU-60019, KU-59403, CP-466722, AZ31, AZ32, AZD0156, and AZD1390, have been developed and their antitumor furnishings take been investigated (Jin and Oh, 2022). Information technology has been reported that human tumor cells treated with KU-55933, a specific inhibitor of the ATM kinase, could sensitize tumor cells to the cytotoxic effects of IR and DNA DSBs-inducing chemotherapeutic agents such every bit etoposide, doxorubicin, and camptothecin (Hickson et al., 2004; Hoey et al., 2022). KU-60019, an improved ATM kinase inhibitor, acts as a highly effective radio-sensitizer in human glioma cells (Biddlestone-Thorpe et al., 2022). AZD0156, a newly discovered ATM inhibitor, has the potential to promote the survival of leukemia-begetting mice and now is under clinical trial (Morgado-Palacin et al., 2022). Preclinical written report demonstrated that ATM inhibitor AZD1390 enhanced the radiosensitivity of tumor cells and extended creature survival in preclinical brain tumor models (Durant et al., 2022). AZD1390, as a radiosensitizer, is at present undergoing two clinical trials in patients with brain cancer (NCT03423628) or non small cell lung cancer (NCT04550104). Many inhibitors aiming at both ATM and DNA-PK have been reported to take slap-up potential as a chemo- and radiotherapy sensitizing agents in cancer therapy (Powell and Bindra, 2009).
The cell cycle checkpoint kinases CHK1 and CHK2 are downstream substrates of ATM /ATR, which act as the "central transducers" of the DDR (Pilie et al., 2022). Activation of these pathways is essential for the proper regulation of checkpoint and Dna repair (Smith et al., 2010). The ATM–Chk2 and ATR–Chk1 pathways respond to different DNA amercement, ATM is activated at DSBs, whereas ATR is recruited to tracts of ssDNA (Di Benedetto et al., 2022). Subsequently, CHK1 and CHK2 activated by ATR and ATM respectively upon their recruitment to DNA damage sites. Protein kinase WEE1 functioned as furthest downstream in ATR/CHK1 pathway, which is indirectly regulated by Dna damage (Cleary et al., 2022). WEE1 actives the G2/K cell cycle checkpoint by impeding cyclin-dependent kinase 1 and 2 (CDK1/2) action, thereby inducing cell cycle arrest and promoting Dna harm repair. Inhibition of WEE1 causes abnormal Deoxyribonucleic acid replication and replication-dependent DNA damage in cells by suppressing CDK2 (Guertin et al., 2022). Recently, compounds targeting CHK1 are currently in clinical trials (Table 1). The showtime-in-class WEE1 kinase inhibitor AZD1775 is also undergoing a series of clinical trials every bit monotherapy or in combination with other therapies (Table i).
mtDNA Repair Pathway
Recently, the exploration of novel anticancer strategies aiming at the differences in mitochondrial role and structure between normal cells and cancer cells has received intensive attention (Porporato et al., 2022). However, in that location are few studies that take discovered new anticancer approaches via targeting mtDNA repair pathway.
Like nDNA, efficient mtDNA repair pathway, specially BER pathway that mainly repairs ROS-induced lesion, may play an important role in cellular resistance to cancer therapeutic agents. MtDNA D-loop mutations were mutual in gastrointestinal cancer and correlated with carcinoma progression (Wang B. et al., 2022). It has been institute that human being chest cancer cells lacking of mtDNA repair are more sensitive to oxidative damage than the control cells (Shokolenko et al., 2003). Grishko 5 I et al indicated that mtDNA repair pathways played an important part in protecting cells against ROS in normal HA1 Chinese hamster fibroblasts (Grishko et al., 2005). Some other study antiseptic that mtDNA repair capacity was important for cellular resistance to oxidative impairment by increasing their viability post-obit exposure to oxidative stress (Shokolenko et al., 2003). Ueta Due east et al demonstrated that downregulation of the mtDNA repair-associated molecules, mitochondrial transcription factor A (mtTFA) and Polγ by using inhibitors of PI3K/Akt signaling in oral squamous cell carcinoma cells (OSC) increased the susceptibility of radio-sensitive OSC cells and radio-resistant OSC cells to gamma-rays (Ueta et al., 2008). This ascertainment implied that PI3K/Akt signal inhibitors tin can suppress mtDNA repair capacity. Thus, these inhibitors combined with ionizing irradiation or chemotherapeutic drugs may be utilized equally an effective strategy in cancer therapy.
DNA glycosylases are involved in the initiation step of BER that recognizes and removes the abnormal base (Anderson and Friedberg, 1980). 8-OxoG-recognizing DNA glycosylase 1 (OGG1) is an important DNA glycosylase for repair of 8-oxoguanine (8-oxoG), which is i of the major DNA lesions both of the nDNA and mtDNA, especially in mtDNA (Rachek et al., 2002). Information technology has been plant that tumor cells harboring overexpressed recombinant OGG1 were more skilful at repairing of oxidative harm to mtDNA, and had increased cellular survival under oxidative stress (Rachek et al., 2002; Yuzefovych et al., 2022). Nosotros previously found that Sirt3, a major mitochondrial NAD+-dependent deacetylase, physically associated with OGG1 and deacetylated this Deoxyribonucleic acid glycosylase, and that deacetylation past Sirt3 prevented the degradation of the OGG1 protein and controlled its incision action (Cheng et al., 2022). Nosotros further showed that regulation of the acetylation and turnover of OGG1 by Sirt3 played a critical role in repairing mitochondrial DNA (mtDNA) harm, protecting mitochondrial integrity, and preventing apoptotic cell death under oxidative stress. We observed that following ionizing radiation, human tumor cells with silencing of Sirt3 expression exhibited oxidative damage of mtDNA, as measured by the aggregating of 8-oxoG and iv,977 mutual deletion, showed more severe mitochondrial dysfunction, and underwent greater apoptosis, in comparing to the cells without silencing of Sirt3 expression. Our results not only reveal a new function and mechanism for Sirt3 in defending the mitochondrial genome against oxidative impairment and in protecting from the genotoxic stress-induced apoptotic prison cell death, but also provide evidence supporting a new mtDNA repair pathway. Recently, researchers also proved that overexpression of mitochondrial OGG1 decreased breast cancer progression and metastasis (Yuzefovych et al., 2022).
In determination, combination of Deoxyribonucleic acid repair pathway inhibitors with anticancer agents may enhance the tumor sensitivity to certain chemotherapeutic drugs and radiation. More than effective and less toxic Deoxyribonucleic acid-damaging agents have been developed and carried out in preclinical studies (Table 2). Based on the preclinical information, a number of clinical trials have been launched to test whether targeting Dna repair pathways tin can reinforce the efficacy of some anticancer drugs and do good cancer patients (Table 1).
The Relationship Between Deoxyribonucleic acid Repair Pathways and Cancer Therapeutic Resistance
Resistance to cancer therapy remains the leading cause of handling failure in cancer patients. DNA repair capacity (DRC) of tumor cells has been known to involve in drug resistance, including chemoradiotherapy, targeted therapy and immunotherapy. Deoxyribonucleic acid damage inducing drug cisplatin is 1 of the near widely employed chemotherapeutic drugs. In a murine model of human lung cancer, tumor cells were initially effective with cisplatin treatment, only resistant emerged afterward prolonged treatment (Oliver et al., 2010). Cisplatin-resistant tumor cells exhibited higher level of Deoxyribonucleic acid impairment repair related genes and DRC, inhibition of NER pathway significantly enhanced the sensitivity of tumor cells to cisplatin (Oliver, Mercer, 2010; Wang et al., 2022). Low expression of 53BP1, a DDR protein involved in NHEJ, was associated with higher local recurrence in triple negative chest cancers (TNBC) patients treated with breast-conserving surgery and radiotherapy, indicating that 53BP1 may be a predictor of radio-resistance (Neboori et al., 2022). PTEN Y240 phosphorylation induced by ionizing radiations (IR), a standard treatment for glioblastoma (GBM) patients, promoted therapeutic resistance by enhancing Dna repair (Ma et al., 2022). Inhibiting DNA repair kinases could besides prevent doxorubicin (DOX) resistance in breast cancer cells (Stefanski et al., 2022). Aberrant DNA repair action was found in CDK4/6 inhibitors palbociclib-resistant breast cancer cells, whereas PARP inhibitors, olaparib and niraparib handling could significantly inhibit palbociclib-resistant cancer cell viability (Kettner et al., 2022). In the contempo years, immunotherapy is a major breakthrough in the field of cancer treatment. Therefore, the office of DDR in tumor immunotherapy has attracted much attention. Studies accept shown deficiency of a specific Dna repair pathway was associated with immune checkpoint blockade (ICB) response. For example, MMR has been reported every bit a disquisitional biomarker of response to immune checkpoint inhibitors in cancer (Le et al., 2022). Alterations in genes encoding MMR proteins ofttimes contribute to frameshift mutations, resulting in neoantigen generation (Germano et al., 2022). Phase II clinical trials proved that mismatch repair–scarce tumors exhibited higher responsive to PD-i blockade compared with mismatch repair–good tumors(Asaoka et al., 2022). Based on lines of pre-clinical and clinical prove, the US Food and drug Assistants (FDA) has approved anti-PD-1 antibodies for the treatment of patients with MMR-deficient (Ruiz-Bañobre and Goel, 2022). On the contrary, researchers also found that colorectal cancer (CRC) patient with Dna mismatch repair deficiency (dMMR)/a high-level of microsatellite instability (MSI-H) exhibited intrinsic resistance to allowed checkpoint immune checkpoint inhibitor (Gurjao et al., 2022). Metastatic urothelial carcinoma (mUC) shown relatively depression response rates to PD-ane/PD-L1 blockade (15–24%), whereas the presence of DDR cistron mutations is a potential marker of clinical do good from anti-PD-1/PD-L1 immune checkpoint inhibitors in mUC (Teo et al., 2022). Preclinical studies have also revealed that suppression of PARP induced PD-L1 expression and consequently caused immunosuppression (Jiao et al., 2022). Researches too elucidated that PARP inhibitor olaparib enhanced CD8+ T-jail cell recruitment and activation by activating the cGAS/STING pathway in BRCA1-deficient triple-negative chest cancer (Pantelidou et al., 2022). Therefore, multiple combination studies involving allowed checkpoint inhibitors with DDR inhibitors are undergoing clinical trials, such every bit combination PARP inhibitor Niraparib and anti-PD-1 antibiotic pembrolizumab in patients with triple-negative breast cancer or ovarian cancer (NCT02657889). In the stage I, multi-eye, dose-escalation report, patients with avant-garde solid tumors will receive WEE1 inhibitor AZD1775 (Adavosertib) in combination with MEDI4736 (durvalumab) (NCT02546661). These studies advise that DRC plays a key part in cancer therapy resistance, therefore, evaluation of DNA repair phenotype before handling could be of great value in clinical direction of clinical therapeutic drugs or modalities.
A number of DDR inhibitors have currently come to market place or nether clinical development. PARP inhibitors are the first clinically approved DDR drugs based on the concept of "synthetic lethal" (Lord and Ashworth, 2022). PARP inhibitors take been widely used for cancer patients with BRCA1/2 mutation or HRR deficiency and showed promising clinical activity. All the same, resistance inevitably adult in the majority of patients and led to treatment failure. The mechanism of resistance to PARP inhibitors can be innate or caused though clinical and preclinical studies. Preclinical studies demonstrated that overexpression of P-glycoprotein drug efflux transporter implicated in intrinsic resistance to Olaparib (Henneman et al., 2022). Resumption of PARformation due to poly (ADP-ribose) glycohydrolase (PARG) depletion conferred acquired resistance to PARP inhibition in BRCA2-deficient tumor cells (Gogola et al., 2022). PARP1 p. T910A mutation could override PARP1 inhibition promoted the secondary failure of Olaparib treatment (Gröschel et al., 2022). Another machinery leading to resistance may restoration of HRR function or re-structure of replication fork stability by increasing RAD51 expression or re-expressing BRCA1/2 (Ter Brugge et al., 2022; Quigley et al., 2022; Clements et al., 2022; Lim et al., 2022; Marzio et al., 2022). Upregulation of certain oncogenic pathways such every bit Wnt/β-catenin signaling pathway or DDR related protein may likewise confer cancer cells insensitive to PARP inhibitors and providing some rationale for combination strategies with PARP inhibitors (Fukumoto et al., 2022; Watson et al., 2022; Liu et al., 2022).
Conclusion and Perspectives
Based on the relationship between Deoxyribonucleic acid repair pathways and cancer development and progression, a new therapeutic strategy has emerged to increase the efficacy of Deoxyribonucleic acid dissentious agents through combination with inhibitors of DNA repair pathways. The inhibitors of several Deoxyribonucleic acid repair pathways have been developed, and some of them are currently undergoing clinical trials. The therapeutic benefits of these agents should exist further evaluated in cancer treatment, and the more specific inhibitors should be developed to reduce the adverse effect on normal tissues and cells. Many studies have demonstrated that the inhibition of Dna repair pathways may be an important manner in anticancer therapies. Yet, nosotros should realize that employ of sure inhibitors of Dna repair pathways may have potential drawbacks. The combination of IR or chemotherapeutic agents with inhibitors of DNA repair pathway may increment the mutagenic lesions in surviving cells and pb to the development of secondary tumors. More attentions have been paid to the relationship between defective nuclear Dna repair pathway and therapeutic resistance but less about the association between the mitochondrial repair pathway and cancer cells. Due to the difference in mtDNA between cancer cells and normal cells, the development of mtDNA repair pathway inhibitors that can reduce the adverse effects to normal cells may exist a more effective strategy to heighten the anticancer therapy than targeting nDNA. A improve understanding on the mechanisms of mtDNA repair pathways shall facilitate the development of new effective chemo- and radiosensitizers past targeting mtDNA repair pathway in cancer therapy.
Author Contributions
LL drafted the manuscript. YG and XC designed the figure and table. JY and YC designed, reviewed, and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Scientific discipline Foundation of China 81422051, 81472593, and 31401208 (YC).
Conflict of Interest
The authors declare that the research was conducted in the absence of whatsoever commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alao, J. P., and Sunnerhagen, P. (2009). The ATM and ATR inhibitors CGK733 and caffeine suppress cyclin D1 levels and inhibit cell proliferation. Radiat. Oncol. iv, 51. doi:10.1186/1748-717x-4-51
PubMed Abstract | CrossRef Total Text | Google Scholar
Alikarami, F., Safa, Thou., Faranoush, G., Hayat, P., and Kazemi, A. (2017). Inhibition of Deoxyribonucleic acid-PK enhances chemosensitivity of B-cell precursor acute lymphoblastic leukemia cells to doxorubicin. Biomed. Pharmacother. 1077–1093. doi:10.1016/j.biopha.2017.08.022
PubMed Abstract | CrossRef Full Text | Google Scholar
Ampferl, R., Rodemann, H. P., Mayer, C., Hofling, T. T. A., and Dittmann, K. (2018). Glucose starvation impairs Dna repair in tumour cells selectively by blocking histone acetylation. Radiother. Oncol. iii, 465–470. doi:10.1016/j.radonc.2017.10.020
PubMed Abstract | CrossRef Total Text | Google Scholar
Anderson, C. T., and Friedberg, Eastward. C. (1980). The presence of nuclear and mitochondrial uracil-Deoxyribonucleic acid glycosylase in extracts of human KB cells. Nucleic Acids Res. four, 875.
PubMed Abstract | Google Scholar
Blindside, Y. J., Xu, R. H., Mentum, K., Lee, Chiliad. W., Park, Due south. H., Rha, S. Y., et al. (2017). Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following offset-line therapy (GOLD): a double-blind, randomised, placebo-controlled, stage 3 trial. Lancet Oncol. 12, 1637–1651. doi:x.1016/s1470-2045(17)30682-4
CrossRef Total Text | Google Scholar
Biddlestone-Thorpe, Fifty., Sajjad, Yard., Rosenberg, E., Beckta, J. M., Valerie, N. C., Tokarz, Grand., et al. (2013). ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin. Cancer Res. 12, 3189–3200. doi:10.1158/1078-0432.ccr-12-3408
PubMed Abstruse | CrossRef Total Text | Google Scholar
Binabaj, M. Grand., Bahrami, A., ShahidSales, S., Joodi, Chiliad., Joudi Mashhad, M., Hassanian, S. Yard., et al. (2018). The prognostic value of MGMT promoter methylation in glioblastoma: a meta-analysis of clinical trials. J. Cell. Physiol. 1, 378–386. doi:ten.1002/jcp.25896
PubMed Abstract | CrossRef Full Text | Google Scholar
Bindra, R. S., Schaffer, P. J., Meng, A., Woo, J., Maseide, Thou., Roth, Chiliad. East., et al. (2004). Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol. Cell Biol. 19, 8504–8518. doi:x.1128/mcb.24.19.8504-8518.2004
CrossRef Full Text | Google Scholar
Bobustuc, Chiliad. C., Patel, A., Thompson, K., Srivenugopal, K. Southward., Frick, J., Weese, J., et al. (2015). MGMT inhibition suppresses survivin expression in pancreatic cancer. Pancreas. iv, 626–635. doi:x.1097/mpa.0000000000000299
CrossRef Full Text | Google Scholar
Brownish, J. Due south., O'Carrigan, B., Jackson, S. P., and Yap, T. A. (2017). Targeting Dna repair in cancer: beyond PARP inhibitors. Canc. Discov. 1, 20–37. doi:10.1158/2159-8290.CD-sixteen-0860
CrossRef Full Text | Google Scholar
Bryant, H. Eastward., Schultz, Due north., Thomas, H. D., Parker, K. M., Bloom, D., Lopez, E., et al. (2005). Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 7035, 913–917. doi:10.1038/nature03443
PubMed Abstruse | CrossRef Full Text | Google Scholar
Carruthers, R., Ahmed, S. U., Strathdee, G., Gomez-Roman, N., Amoah-Buahin, Due east., Watts, C., et al. (2015). Absconding of radioresistance in glioblastoma stem-similar cells past inhibition of ATM kinase. Mol. Oncol. 1, 192–203. doi:ten.1016/j.molonc.2014.08.003
CrossRef Full Text | Google Scholar
Cerrato, A., Morra, F., and Celetti, A. (2016). Utilise of poly ADP-ribose polymerase [PARP] inhibitors in cancer cells begetting DDR defects: the rationale for their inclusion in the clinic. J. Exp. Clin. Cancer Res. i, 179. doi:x.1186/s13046-016-0456-two
PubMed Abstract | CrossRef Total Text | Google Scholar
Cheng, Y., Ren, 10., Gowda, A. S., Shan, Y., Zhang, 50., Yuan, Y. Southward., et al. (2013). Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial Dna and protects from apoptotic cell death nether oxidative stress. Jail cell Decease Dis. 4, e731. doi:10.1038/cddis.2013.254
PubMed Abstract | CrossRef Total Text | Google Scholar
Chowdhury, S. 1000., Surhland, C., Sanchez, Z., Chaudhary, P., Suresh Kumar, M. A., Lee, S., et al. (2015). Graphene nanoribbons equally a drug delivery amanuensis for lucanthone mediated therapy of glioblastoma multiforme. Nanomedicine 1, 109–118. doi:10.1016/j.nano.2014.08.001
CrossRef Full Text | Google Scholar
Ciszewski, Westward. M., Tavecchio, M., Dastych, J., and Curtin, Due north. J. (2014). Dna-PK inhibition by NU7441 sensitizes breast cancer cells to ionizing radiations and doxorubicin. Breast Cancer Res. Care for i, 47–55. doi:x.1007/s10549-013-2785-6
CrossRef Full Text | Google Scholar
Cleary, J. Thousand., Aguirre, A. J., Shapiro, One thousand. I., and D'Andrea, A. D. (2020). Biomarker-guided evolution of Deoxyribonucleic acid repair inhibitors. Mol. Cell. half dozen, 1070–1085. doi:10.1016/j.molcel.2020.04.035
PubMed Abstruse | CrossRef Full Text | Google Scholar
Clementi, Eastward., Inglin, L., Beebe, E., Gsell, C., Garajova, Z., and Markkanen, E. (2020). Persistent Dna damage triggers activation of the integrated stress response to promote cell survival under nutrient brake. BMC Biol 1, 36. doi:10.1186/s12915-020-00771-x
CrossRef Full Text | Google Scholar
Clements, G. E., Thakar, T., Nicolae, C. Yard., Liang, X., Wang, H. K., and Moldovan, Grand. 50. (2018). Loss of E2F7 confers resistance to poly-ADP-ribose polymerase (PARP) inhibitors in BRCA2-deficient cells. Nucleic Acids Res. 17, 8898–8907. doi:ten.1093/nar/gky657
PubMed Abstruse | CrossRef Full Text | Google Scholar
Coleman, R. Fifty., Fleming, G. F., Brady, M. F., Swisher, Due east. Chiliad., Steffensen, K. D., Friedlander, G., et al. (2019). Veliparib with showtime-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 25, 2403–2415. doi:10.1056/NEJMoa1909707
PubMed Abstract | CrossRef Full Text | Google Scholar
Dhillon, K. K., Bajrami, I., Taniguchi, T., and Lord, C. J. (2016). Synthetic lethality: the route to novel therapies for breast cancer. Endocr. Relat. Cancer. x, T39–T55. doi:10.1530/erc-16-0228
PubMed Abstract | CrossRef Total Text | Google Scholar
Di Benedetto, A., Ercolani, C., Mottolese, M., Sperati, F., Pizzuti, 50., Vici, P., et al. (2017). Assay of the ATR-Chk1 and ATM-Chk2 pathways in male breast cancer revealed the prognostic significance of ATR expression. Sci. Rep. one, 8078. doi:ten.1038/s41598-017-07366-7
PubMed Abstract | CrossRef Total Text | Google Scholar
Dolman, M. East., van der Ploeg, I., Koster, J., Bate-Eya, Fifty. T., Versteeg, R., Caron, H. N., et al. (2015). DNA-dependent poly peptide kinase equally molecular target for radiosensitization of neuroblastoma cells. PLoS One. 12, e0145744. doi:10.1371/journal.pone.0145744
PubMed Abstract | CrossRef Full Text | Google Scholar
Durant, Southward. T., Zheng, L., Wang, Y., Chen, K., Zhang, L., Zhang, T., et al. (2018). The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci. Adv. half dozen, eaat1719. doi:10.1126/sciadv.aat1719
PubMed Abstruse | CrossRef Total Text | Google Scholar
Erice, O., Smith, Grand. P., White, R., Goicoechea, I., Barriuso, J., Jones, C., et al. (2015). MGMT expression predicts PARP-mediated resistance to temozolomide. Mol. Cancer Ther. 5, 1236–1246. doi:10.1158/1535-7163.mct-14-0810
PubMed Abstract | CrossRef Total Text | Google Scholar
Farago, A. F., Yeap, B. Y., Stanzione, M., Hung, Y. P., Heist, R. S., Marcoux, J. P., et al. (2019). Combination olaparib and temozolomide in relapsed small-prison cell lung cancer. Cancer Discov. x, 1372–1387. doi:10.1158/2159-8290.cd-nineteen-0582
PubMed Abstruse | CrossRef Total Text | Google Scholar
Farmer, H., McCabe, N., Lord, C. J., Tutt, A. N., Johnson, D. A., Richardson, T. B., et al. (2005). Targeting the Deoxyribonucleic acid repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 7035, 917–921. doi:10.1038/nature03445
PubMed Abstract | CrossRef Full Text | Google Scholar
Fishel, M. L., Xia, H., McGeown, J., McIlwain, D. Westward., Elbanna, Thou., Arts and crafts, A. A., et al. (2019). Antitumor activity and mechanistic characterization of APE1/ref-1 inhibitors in bladder cancer. Mol. Cancer Ther. eleven, 1947–1960. doi:10.1158/1535-7163.mct-18-1166
PubMed Abstract | CrossRef Full Text | Google Scholar
Foote, M. K., Blades, K., Cronin, A., Fillery, S., Guichard, Southward. Southward., Hassall, 50., et al. (2013). Discovery of four-{iv-[(3R)-3-Methylmorpholin-four-yl]-6-[1-(methylsulfonyl)cyclopropyl]pyrimidin-2-y l}-1H-indole (AZ20): a potent and selective inhibitor of ATR protein kinase with monotherapy in vivo antitumor activity. J. Med. Chem. five, 2125–2138. doi:x.1021/jm301859s
PubMed Abstract | CrossRef Total Text | Google Scholar
Fukumoto, T., Zhu, H., Nacarelli, T., Karakashev, South., Fatkhutdinov, N., Wu, South., et al. (2019). N(6)-Methylation of adenosine of FZD10 mRNA contributes to PARP inhibitor resistance. Cancer Res. 11, 2812–2820. doi:x.1158/0008-5472.tin can-xviii-3592
PubMed Abstract | CrossRef Total Text | Google Scholar
Gao, X., Wang, J., Li, Chiliad., Wang, J., Lv, J., Zhang, L., et al. (2019). Berberine attenuates XRCC1-mediated base excision repair and sensitizes breast cancer cells to the chemotherapeutic drugs. J. Cell Mol. Med. x, 6797–6804. doi:10.1111/jcmm.14560
PubMed Abstruse | CrossRef Full Text | Google Scholar
Germano, Chiliad., Lamba, S., Rospo, Thousand., Barault, L., Magrì, A., Maione, F., et al. (2017). Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 7683, 116–120. doi:10.1038/nature24673
PubMed Abstract | CrossRef Full Text | Google Scholar
Gogola, E., Duarte, A. A., de Ruiter, J. R., Wiegant, W. W., Schmid, J. A., de Bruijn, R., et al. (2018). Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell. half-dozen, 1078–1093.e12. doi:10.1016/j.ccell.2018.05.008
CrossRef Full Text | Google Scholar
Golan, T., Hammel, P., Reni, M., Van Cutsem, Eastward., Macarulla, T., Hall, M. J., et al. (2019). Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. North. Engl. J. Med. 4, 317–327. doi:10.1056/NEJMoa1903387
PubMed Abstruse | CrossRef Total Text | Google Scholar
Grishko, Five. I., Rachek, 50. I., Spitz, D. R., Wilson, G. L., and LeDoux, S. P. (2005). Contribution of mitochondrial DNA repair to cell resistance from oxidative stress. J. Biol. Chem. 10, 8901–8905. doi:10.1074/jbc.M413022200
PubMed Abstract | CrossRef Full Text | Google Scholar
Gröschel, Southward., Hübschmann, D., Raimondi, F., Horak, P., Warsow, Thou., Fröhlich, M., et al. (2019). Lacking homologous recombination DNA repair as therapeutic target in advanced chordoma. Nat. Commun. one, 1635. doi:10.1038/s41467-019-09633-9
CrossRef Total Text | Google Scholar
Guertin, A. D., Li, J., Liu, Y., Hurd, Chiliad. S., Schuller, A. G., Long, B., et al. (2013). Preclinical evaluation of the WEE1 inhibitor MK-1775 every bit unmarried-amanuensis anticancer therapy. Mol. Cancer Ther. 8, 1442–1452. doi:ten.1158/1535-7163.MCT-13-0025
PubMed Abstract | CrossRef Full Text | Google Scholar
Gurjao, C., Liu, D., Hofree, M., AlDubayan, S. H., Wakiro, I., Su, M. J., et al. (2019). Intrinsic resistance to immune checkpoint blockade in a mismatch repair-deficient colorectal cancer. Cancer Immunol. Res. 8, 1230–1236. doi:10.1158/2326-6066.CIR-xviii-0683
PubMed Abstract | CrossRef Full Text | Google Scholar
Hampel, H., Frankel, W. 50., Martin, Due east., Arnold, Yard., Khanduja, K., Kuebler, P., et al. (2005). Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N. Engl. J. Med. eighteen, 1851–1860. doi:10.1056/NEJMoa043146
PubMed Abstract | CrossRef Full Text | Google Scholar
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell. five, 646–674. doi:10.1016/j.cell.2011.02.013
CrossRef Full Text | Google Scholar
Happold, C., Stojcheva, Northward., Silginer, K., Weiss, T., Roth, P., Reifenberger, Chiliad., et al. (2018). Transcriptional control of O(6) -methylguanine Deoxyribonucleic acid methyltransferase expression and temozolomide resistance in glioblastoma. J. Neurochem. 6, 780–790. doi:10.1111/jnc.14326
CrossRef Total Text | Google Scholar
Hegi, Chiliad. Due east., Diserens, A. C., Gorlia, T., Hamou, One thousand. F., de Tribolet, N., Weller, 1000., et al. (2005). MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. x, 997–1003. doi:10.1056/NEJMoa043331
PubMed Abstract | CrossRef Full Text | Google Scholar
Henneman, Fifty., van Miltenburg, Chiliad. H., Michalak, E. M., Braumuller, T. M., Jaspers, J. E., Drenth, A. P., et al. (2015). Selective resistance to the PARP inhibitor olaparib in a mouse model for BRCA1-deficient metaplastic breast cancer. Proc. Natl. Acad. Sci. UsA. 27, 8409–8414. doi:ten.1073/pnas.1500223112
PubMed Abstract | CrossRef Full Text | Google Scholar
Hickson, I., Zhao, Y., Richardson, C. J., Green, South. J., Martin, Due north. M., Orr, A. I., et al. (2004). Identification and label of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 24, 9152–9159. doi:10.1158/0008-5472.tin-04-2727
PubMed Abstruse | CrossRef Full Text | Google Scholar
Hoey, C., Ray, J., Jeon, J., Huang, X., Taeb, South., Ylanko, J., et al. (2018). miRNA-106a and prostate cancer radioresistance: a novel role for LITAF in ATM regulation. Mol. Oncol. 8, 1324–1341. doi:ten.1002/1878-0261.12328
PubMed Abstract | CrossRef Full Text | Google Scholar
Hosoya, N., and Miyagawa, Grand. (2014). Targeting DNA impairment response in cancer therapy. Cancer Sci. 4, 370–388. doi:10.1111/cas.12366
CrossRef Full Text | Google Scholar
Hsu, S. H., Chen, S. H., Kuo, C. C., and Chang, J. Y. (2018). Ubiquitin-conjugating enzyme E2 B regulates the ubiquitination of O(6)-methylguanine-Deoxyribonucleic acid methyltransferase and BCNU sensitivity in human nasopharyngeal carcinoma cells. Biochem. Pharmacol. 158, 327–338. doi:10.1016/j.bcp.2018.10.029
PubMed Abstract | CrossRef Full Text | Google Scholar
Huang, F., and Mazin, A. Five. (2014). A modest molecule inhibitor of homo RAD51 potentiates breast cancer cell killing by therapeutic agents in mouse xenografts. PLoS 1. 6, e100993. doi:ten.1371/journal.pone.0100993
PubMed Abstract | CrossRef Full Text | Google Scholar
Iaccarino, C., Orlandi, E., Ruggeri, F., Nicoli, D., Torricelli, F., Maggi, One thousand., et al. (2015). Prognostic value of MGMT promoter condition in not-resectable glioblastoma after adjuvant therapy. Clin. Neurol. Neurosurg. 132, 1–8. doi:10.1016/j.clineuro.2015.01.029
PubMed Abstruse | CrossRef Full Text | Google Scholar
Jette, N., and Lees-Miller, S. P. (2015). The DNA-dependent protein kinase: a multifunctional protein kinase with roles in Dna double strand pause repair and mitosis. Prog. Biophys. Mol. Biol. 2-three, 194–205. doi:10.1016/j.pbiomolbio.2014.12.003
CrossRef Full Text | Google Scholar
Jiao, South., Xia, W., Yamaguchi, H., Wei, Y., Chen, M. K., Hsu, J. Thousand., et al. (2017). PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin. Cancer Res. xiv, 3711–3720. doi:10.1158/1078-0432.ccr-16-3215
PubMed Abstract | CrossRef Full Text | Google Scholar
Jin, M. H., and Oh, D. Y. (2019). ATM in DNA repair in cancer. Pharmacol. Ther. [Epub ahead of print]. doi:10.1016/j.pharmthera.2019.07.002
CrossRef Full Text | Google Scholar
Jue, T. R., Nozue, K., Lester, A. J., Joshi, Due south., Schroder, 50. B., Whittaker, S. P., et al. (2017). Veliparib in combination with radiotherapy for the handling of MGMT unmethylated glioblastoma. J. Transl. Med. 1, 61. doi:10.1186/s12967-017-1164-1
CrossRef Full Text | Google Scholar
Kaufman, B., Shapira-Frommer, R., Schmutzler, R. K., Audeh, 1000. W., Friedlander, M., Balmana, J., et al. (2015). Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. three, 244–250. doi:10.1200/jco.2014.56.2728
CrossRef Full Text | Google Scholar
Kettner, Due north. Grand., Vijayaraghavan, South., Durak, M. 1000., Bui, T., Kohansal, One thousand., Ha, Thou. J., et al. (2019). Combined inhibition of STAT3 and DNA repair in palbociclib-resistant ER-positive breast cancer. Clin. Cancer Res. xiii, 3996–4013. doi:10.1158/1078-0432.ccr-18-3274
PubMed Abstruse | CrossRef Total Text | Google Scholar
Kim, G., Ison, Yard., McKee, A. Eastward., Zhang, H., Tang, S., Gwise, T., et al. (2015). FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated avant-garde ovarian cancer treated with 3 or more lines of chemotherapy. Clin. Cancer Res. 19, 4257–4261. doi:x.1158/1078-0432.ccr-fifteen-0887
CrossRef Total Text | Google Scholar
Kim, Y. S., Kim, S. H., Cho, J., Kim, J. W., Chang, J. H., Kim, D. South., et al. (2012). MGMT gene promoter methylation every bit a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a unmarried-institution study. Int. J. Radiat. Oncol. Biol. Phys. 3, 661–667. doi:10.1016/j.ijrobp.2011.12.086
CrossRef Full Text | Google Scholar
King, H. O., Brend, T., Payne, H. L., Wright, A., Ward, T. A., Patel, K., et al. (2017). RAD51 is a selective Dna repair target to radiosensitize glioma stem cells. Stem Prison cell Rep. 1, 125–139. doi:x.1016/j.stemcr.2016.12.005
CrossRef Full Text | Google Scholar
Konecny, G. E., and Kristeleit, R. Southward. (2016). PARP inhibitors for BRCA1/2-mutated and sporadic ovarian cancer: current do and time to come directions. Br. J. Cancer. ten, 1157–1173. doi:10.1038/bjc.2016.311
PubMed Abstract | CrossRef Full Text | Google Scholar
Konstantinopoulos, P. A., Barry, W. T., Birrer, Thousand., Westin, Due south. N., Cadoo, K. A., Shapiro, G. I., et al. (2019). Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. iv, 570–580. doi:10.1016/s1470-2045(eighteen)30905-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Koosha, F., Neshasteh-Riz, A., Takavar, A., Eyvazzadeh, N., Mazaheri, Z., Eynali, S., et al. (2017). The combination of A-966492 and Topotecan for effective radiosensitization on glioblastoma spheroids. Biochem. Biophys. Res. Commun. 4, 1092–1097. doi:10.1016/j.bbrc.2017.08.018
PubMed Abstruse | CrossRef Total Text | Google Scholar
Le, D. T., Durham, J. N., Smith, K. N., Wang, H., Bartlett, B. R., Aulakh, 50. Yard., et al. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 6349, 409–413. doi:10.1126/science.aan6733
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, A., Youssef, I., Osborn, V. W., Safdieh, J., Becker, D. J., and Schreiber, D. (2018). The utilization of MGMT promoter methylation testing in United states hospitals for glioblastoma and its impact on prognosis. J. Clin. Neurosci. 51, 85–90. doi:10.1016/j.jocn.2018.02.009
PubMed Abstract | CrossRef Full Text | Google Scholar
Lim, M. S., Li, H., Roberts, Eastward. A., Gaudiano, E. F., Clairmont, C., Sambel, Fifty. A., et al. (2018). USP1 is required for replication fork protection in BRCA1-deficient tumors. Mol. Cell. vi, 925–941.e4. doi:10.1016/j.molcel.2018.x.045
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu, J. F., Barry, Due west. T., Birrer, Thou., Lee, J. M., Buckanovich, R. J., Fleming, K. F., et al. (2019). Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann. Oncol. 4, 551–557. doi:10.1093/annonc/mdz018
CrossRef Total Text | Google Scholar
Liu, 50., Cai, S., Han, C., Banerjee, A., Wu, D., Cui, T., et al. (2020). ALDH1A1 contributes to PARP inhibitor resistance via enhancing DNA repair in BRCA2(-/-) ovarian cancer cells. Mol. Cancer Ther. 1, 199–210. doi:10.1158/1535-7163.mct-nineteen-0242
CrossRef Full Text | Google Scholar
Liu, L., Yan, L., Donze, J. R., and Gerson, Southward. Fifty. (2003). Blockage of abasic site repair enhances antitumor efficacy of 1,3-bis-(2-chloroethyl)-1-nitrosourea in colon tumor xenografts. Mol. Cancer Ther. 10, 1061
PubMed Abstract | Google Scholar
Liu, Y., Zhang, L., Liu, Y., Lord's day, C., Zhang, H., Miao, G., et al. (2015). Deoxyribonucleic acid-PKcs deficiency inhibits glioblastoma cell-derived angiogenesis after ionizing radiation. J. Cell. Physiol. 5, 1094–1103. doi:x.1002/jcp.24841
PubMed Abstruse | CrossRef Total Text | Google Scholar
Loibl, S., O'Shaughnessy, J., Untch, M., Sikov, W. Thou., Rugo, H. Southward., McKee, M. D., et al. (2018). Improver of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, stage 3 trial. Lancet Oncol. iv, 497–509. doi:10.1016/s1470-2045(xviii)30111-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Lu, Y., Chu, A., Turker, K. Southward., and Glazer, P. M. (2011). Hypoxia-induced epigenetic regulation and silencing of the BRCA1 promoter. Mol. Cell Biol. 16, 3339–3350. doi:x.1128/mcb.01121-10
PubMed Abstract | CrossRef Full Text | Google Scholar
Luo, 1000., and Kelley, Thousand. R. (2004). Inhibition of the homo apurinic/apyrimidinic endonuclease (APE1) repair activeness and sensitization of breast cancer cells to DNA alkylating agents with lucanthone. Anticancer Res. iv, 2127
PubMed Abstruse | Google Scholar
Ma, H., Takahashi, A., Yoshida, Y., Adachi, A., Kanai, T., Ohno, T., et al. (2015). Combining carbon ion irradiation and non-homologous end-joining repair inhibitor NU7026 efficiently kills cancer cells. Radiat. Oncol. 10, 225. doi:10.1186/s13014-015-0536-z
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ma, J., Benitez, J. A., Li, J., Miki, Due south., Ponte de Albuquerque, C., Galatro, T., et al. (2019). Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell. 3, 504–518.e7. doi:10.1016/j.ccell.2019.01.020
PubMed Abstract | CrossRef Full Text | Google Scholar
Maki, Y., Murakami, J., Asaumi, J., Tsujigiwa, H., Nagatsuka, H., Kokeguchi, Due south., et al. (2005). Part of O6-methylguanine-Deoxyribonucleic acid methyltransferase and consequence of O6-benzylguanine on the anti-tumor activity of cis-diaminedichloroplatinum(II) in oral cancer jail cell lines. Oral Oncol. x, 984–993. doi:10.1016/j.oraloncology.2005.05.011
PubMed Abstract | CrossRef Full Text | Google Scholar
Marzio, A., Puccini, J., Kwon, Y., Maverakis, N. Chiliad., Arbini, A., Sung, P., et al. (2019). The F-box domain-dependent activity of EMI1 regulates PARPi sensitivity in triple-negative breast cancers. Mol. Cell. ii, 224–237.e6. doi:x.1016/j.molcel.2018.xi.003
CrossRef Full Text | Google Scholar
Memon, D., Dawson, Thou., Smowton, C. S., Xing, W., Dive, C., and Miller, C. J. (2016). Hypoxia-driven splicing into noncoding isoforms regulates the Deoxyribonucleic acid damage response. NPJ Genom. Med. one, 16020. doi:10.1038/npjgenmed.2016.20
PubMed Abstruse | CrossRef Full Text | Google Scholar
Middleton, M. R., Thatcher, N., McMurry, T. B., McElhinney, R. S., Donnelly, D. J., and Margison, G. P. (2002). Effect of O6-(4-bromothenyl)guanine on different temozolomide schedules in a human being melanoma xenograft model. Int. J. Cancer. 5, 615–617. doi:10.1002/ijc.10532
CrossRef Full Text | Google Scholar
Mihaylova, V. T., Bindra, R. Southward., Yuan, J., Campisi, D., Narayanan, L., Jensen, R., et al. (2003). Decreased expression of the DNA mismatch repair cistron Mlh1 under hypoxic stress in mammalian cells. Mol. Prison cell Biol. 9, 3265–3273. doi:10.1128/mcb.23.nine.3265-3273.2003
PubMed Abstract | CrossRef Full Text | Google Scholar
Montaldi, A. P., and Sakamoto-Hojo, E. T. (2013). Methoxyamine sensitizes the resistant glioblastoma T98G cell line to the alkylating agent temozolomide. Clin. Exp. Med. 4, 279–288. doi:x.1007/s10238-012-0201-10
CrossRef Total Text | Google Scholar
Moore, K., Colombo, N., Scambia, G., Kim, B. G., Oaknin, A., Friedlander, Yard., et al. (2018). Maintenance olaparib in patients with newly diagnosed avant-garde ovarian cancer. North. Engl. J. Med. 26, 2495–2505. doi:ten.1056/NEJMoa1810858
PubMed Abstract | CrossRef Full Text | Google Scholar
Morgado-Palacin, I., Day, A., Murga, M., Lafarga, 5., Anton, M. E., Tubbs, A., et al. (2016). Targeting the kinase activities of ATR and ATM exhibits antitumoral activity in mouse models of MLL-rearranged AML. Sci. Bespeak. 445, ra91. doi:10.1126/scisignal.aad8243
PubMed Abstruse | CrossRef Full Text | Google Scholar
Muralidharan, S. 5., Bhadury, J., Nilsson, L. One thousand., Green, L. C., McLure, G. G., and Nilsson, J. A. (2016). BET bromodomain inhibitors synergize with ATR inhibitors to induce DNA impairment, apoptosis, senescence-associated secretory pathway and ER stress in Myc-induced lymphoma cells. Oncogene. 36, 4689–4697. doi:10.1038/onc.2015.521
PubMed Abstract | CrossRef Full Text | Google Scholar
Neboori, H. J., Haffty, B. G., Wu, H., Yang, Q., Aly, A., Goyal, Southward., et al. (2012). Low p53 bounden protein 1 (53BP1) expression is associated with increased local recurrence in chest cancer patients treated with chest-conserving surgery and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. five, e677–83. doi:10.1016/j.ijrobp.2012.01.089
PubMed Abstruse | CrossRef Total Text | Google Scholar
Neijenhuis, S., Begg, A. C., and Vens, C. (2005). Radiosensitization by a ascendant negative to Deoxyribonucleic acid polymerase beta is DNA polymerase beta-independent and XRCC1-dependent. Radiother. Oncol. 2, 123–128. doi:ten.1016/j.radonc.2005.06.020
PubMed Abstract | CrossRef Total Text | Google Scholar
Ohta, S. (2006). Contribution of somatic mutations in the mitochondrial genome to the development of cancer and tolerance against anticancer drugs. Oncogene. 34, 4768–4776. doi:10.1038/sj.onc.1209602
PubMed Abstruse | CrossRef Full Text | Google Scholar
Oleinick, N. 50., Biswas, T., Patel, R., Tao, M., Patel, R., Weeks, L., et al. (2016). Radiosensitization of non-small-cell lung cancer cells and xenografts past the interactive effects of pemetrexed and methoxyamine. Radiother. Oncol. 2, 335–341. doi:10.1016/j.radonc.2016.10.007
PubMed Abstract | CrossRef Full Text | Google Scholar
Oliver, T. G., Mercer, Thou. 50., Sayles, L. C., Shush, J. R., Mendus, D., Lovejoy, 1000. S., et al. (2010). Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Genes Dev. 8, 837–852. doi:10.1101/gad.1897010
PubMed Abstract | CrossRef Full Text | Google Scholar
Oplustil O'Connor, 50., Rulten, Due south. L., Cranston, A. N., Odedra, R., Chocolate-brown, H., Jaspers, J. Eastward., et al. (2016). The PARP inhibitor AZD2461 provides insights into the role of PARP3 inhibition for both synthetic lethality and tolerability with chemotherapy in preclinical models. Cancer Res. 20, 6084–6094. doi:ten.1158/0008-5472.CAN-15-3240
CrossRef Full Text | Google Scholar
Pantelidou, C., Sonzogni, O., De Oliveria Taveira, M., Mehta, A. Yard., Kothari, A., Wang, D., et al. (2019). PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 6, 722–737. doi:10.1158/2159-8290.cd-eighteen-1218
PubMed Abstract | CrossRef Full Text | Google Scholar
Pearl, L. H., Schierz, A. C., Ward, South. E., Al-Lazikani, B., and Pearl, F. Grand. (2015). Therapeutic opportunities inside the Dna impairment response. Nat. Rev. Cancer. 3, 166–180. doi:x.1038/nrc3891
PubMed Abstract | CrossRef Full Text | Google Scholar
Pilie, P. G., Tang, C., Mills, G. B., and Yap, T. A. (2019). State-of-the-fine art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2, 81–104. doi:10.1038/s41571-018-0114-z
PubMed Abstruse | CrossRef Full Text | Google Scholar
Porporato, P. East., Filigheddu, Due north., Pedro, J. M. B., Kroemer, G., and Galluzzi, 50. (2018). Mitochondrial metabolism and cancer. Cell Res. 3, 265–280. doi:ten.1038/cr.2017.155
CrossRef Full Text | Google Scholar
Quigley, D., Alumkal, J. J., Wyatt, A. W., Kothari, V., Foye, A., Lloyd, P., et al. (2017). Analysis of circulating prison cell-free Deoxyribonucleic acid identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. ix, 999–1005. doi:10.1158/2159-8290.cd-17-0146
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rabik, C. A., Njoku, M. C., and Dolan, M. E. (2006). Inactivation of O6-alkylguanine DNA alkyltransferase equally a ways to enhance chemotherapy. Cancer Treat Rev. 4, 261–276. doi:10.1016/j.ctrv.2006.03.004
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rachek, 50. I., Grishko, V. I., Musiyenko, S. I., Kelley, M. R., LeDoux, S. P., and Wilson, G. L. (2002). Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J. Biol. Chem. 47, 44932–44937. doi:10.1074/jbc.M208770200
PubMed Abstract | CrossRef Full Text | Google Scholar
Ranson, Thou., Middleton, M. R., Bridgewater, J., Lee, S. M., Dawson, One thousand., Jowle, D., et al. (2006). Lomeguatrib, a strong inhibitor of O6-alkylguanine-DNA-alkyltransferase: stage I safe, pharmacodynamic, and pharmacokinetic trial and evaluation in combination with temozolomide in patients with advanced solid tumors. Clin. Cancer Res. 5, 1577–1584. doi:x.1158/1078-0432.ccr-05-2198
PubMed Abstract | CrossRef Full Text | Google Scholar
Riaz, N., Blecua, P., Lim, R. S., Shen, R., Higginson, D. S., Weinhold, N., et al. (2017). Pan-cancer analysis of bi-allelic alterations in homologous recombination Deoxyribonucleic acid repair genes. Nat. Commun. ane, 857. doi:10.1038/s41467-017-00921-w
PubMed Abstract | CrossRef Full Text | Google Scholar
Robson, M., Im, S. A., Senkus, E., Xu, B., Domchek, Southward. Yard., Masuda, Due north., et al. (2017). Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. Due north. Engl. J. Med. half-dozen, 523–533. doi:10.1056/NEJMoa1706450
PubMed Abstract | CrossRef Full Text | Google Scholar
Ruiz-Bañobre, J., and Goel, A. (2019). Deoxyribonucleic acid mismatch repair deficiency and allowed checkpoint inhibitors in gastrointestinal cancers. Gastroenterology. 4, 890–903. doi:10.1053/j.gastro.2018.11.071
CrossRef Total Text | Google Scholar
Saki, M., and Prakash, A. (2017). DNA damage related crosstalk betwixt the nucleus and mitochondria. Costless Radic. Biol. Med. 216–227. doi:10.1016/j.freeradbiomed.2016.11.050
CrossRef Full Text | Google Scholar
Seo, Y., and Kinsella, T. J. (2009). Essential office of Deoxyribonucleic acid base excision repair on survival in an acidic tumor microenvironment. Cancer Res. 18, 7285–7293. doi:10.1158/0008-5472.can-09-0624
PubMed Abstruse | CrossRef Total Text | Google Scholar
Shi, Q., Maas, 50., Veith, C., Van Schooten, F. J., and Godschalk, R. W. (2017). Acidic cellular microenvironment modifies carcinogen-induced DNA damage and repair. Arch. Toxicol. 6, 2425–2441. doi:10.1007/s00204-016-1907-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Shi, Y., Wang, Y., Qian, J., Yan, 10., Han, Y., Yao, N., et al. (2020). MGMT expression affects the gemcitabine resistance of pancreatic cancer cells. Life Sci. 259, 118148. doi:ten.1016/j.lfs.2020.118148
PubMed Abstruse | CrossRef Full Text | Google Scholar
Shinohara, Due east. T., Geng, L., Tan, J., Chen, H., Shir, Y., Edwards, E., et al. (2005). Deoxyribonucleic acid-dependent protein kinase is a molecular target for the development of noncytotoxic radiation-sensitizing drugs. Cancer Res. 12, 4987–4992. doi:ten.1158/0008-5472.can-04-4250
PubMed Abstract | CrossRef Full Text | Google Scholar
Shokolenko, I. Northward., Alexeyev, M. F., Robertson, F. Yard., LeDoux, S. P., and Wilson, Chiliad. L. (2003). The expression of Exonuclease 3 from Due east. coli in mitochondria of breast cancer cells diminishes mitochondrial DNA repair capacity and cell survival after oxidative stress. Dna Repair. 2, 471–482. doi:ten.1016/s1568-7864(03)00019-3
PubMed Abstract | CrossRef Total Text | Google Scholar
Smith, J., Tho, L. M., Xu, N., and Gillespie, D. A. (2010). The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 73–112. doi:x.1016/b978-0-12-380888-2.00003-0
CrossRef Full Text | Google Scholar
Squatrito, Chiliad., Brennan, C. W., Helmy, Grand., Huse, J. T., Petrini, J. H., and The netherlands, East. C. (2010). Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell. 6, 619–629. doi:10.1016/j.ccr.2010.10.034
PubMed Abstract | CrossRef Total Text | Google Scholar
Stefanski, C. D., Keffler, Thou., McClintock, Due south., Milac, L., and Prosperi, J. R. (2019). APC loss affects Deoxyribonucleic acid damage repair causing doxorubicin resistance in breast cancer cells. Neoplasia. 12, 1143–1150. doi:ten.1016/j.neo.2019.09.002
CrossRef Full Text | Google Scholar
Sunada, Southward., Kanai, H., Lee, Y., Yasuda, T., Hirakawa, H., Liu, C., et al. (2016). Nontoxic concentration of DNA-PK inhibitor NU7441 radio-sensitizes lung tumor cells with little effect on double strand intermission repair. Cancer Sci. 9, 1250–1255. doi:10.1111/cas.12998
PubMed Abstract | CrossRef Full Text | Google Scholar
Teng, P. N., Bateman, Northward. Westward., Darcy, K. G., Hamilton, C. A., Maxwell, G. L., Bakkenist, C. J., et al. (2015). Pharmacologic inhibition of ATR and ATM offers clinically important distinctions to enhancing platinum or radiations response in ovarian, endometrial, and cervical cancer cells. Gynecol. Oncol. 3, 554–561. doi:10.1016/j.ygyno.2014.12.035
PubMed Abstruse | CrossRef Full Text | Google Scholar
Teo, G. Y., Seier, K., Ostrovnaya, I., Regazzi, A. M., Kania, B. East., Moran, M. M., et al. (2018). Alterations in DNA damage response and repair genes every bit potential marker of clinical benefit from PD-i/PD-L1 blockade in advanced urothelial cancers. J. Clin. Oncol. 17, 1685–1694. doi:10.1200/jco.2017.75.7740
PubMed Abstract | CrossRef Full Text | Google Scholar
Ter Brugge, P., Kristel, P., van der Burg, E., Benefaction, U., de Maaker, M., Lips, E., et al. (2016). Mechanisms of therapy resistance in patient-derived xenograft models of BRCA1-deficient breast cancer. J. Natl. Cancer Inst. 112, 1075. doi:ten.1093/jnci/djw148
CrossRef Full Text | Google Scholar
Tian, H., Gao, Z., Li, H., Zhang, B., Wang, G., Zhang, Q., et al. (2015). DNA damage response--a double-edged sword in cancer prevention and cancer therapy. Cancer Lett. 1, 8–16. doi:ten.1016/j.canlet.2014.12.038
CrossRef Total Text | Google Scholar
Tran, T. Q., Ishak Gabra, M. B., Lowman, X. H., Yang, Y., Reid, K. A., Pan, One thousand., et al. (2017). Glutamine deficiency induces Deoxyribonucleic acid alkylation impairment and sensitizes cancer cells to alkylating agents through inhibition of ALKBH enzymes. PLoS Biol. xi, e2002810. doi:10.1371/journal.pbio.2002810
PubMed Abstract | CrossRef Full Text | Google Scholar
Tsai, A. Grand., Khan, A. Y., Worgo, C. E., Wang, L. Fifty., Liang, Y., and Davila, E. (2017). A multikinase and Deoxyribonucleic acid-PK inhibitor combination immunomodulates melanomas, suppresses tumor progression, and enhances immunotherapies. Cancer Immunol. Res. 9, 790–803. doi:10.1158/2326-6066.CIR-17-0009
PubMed Abstract | CrossRef Total Text | Google Scholar
Ueta, Eastward., Sasabe, Due east., Yang, Z., Osaki, T., and Yamamoto, T. (2008). Enhancement of apoptotic harm of squamous cell carcinoma cells by inhibition of the mitochondrial Deoxyribonucleic acid repairing system. Cancer Sci. 11, 2230–2237. doi:10.1111/j.1349-7006.2008.00918.10
PubMed Abstract | CrossRef Total Text | Google Scholar
Vazquez-Ortiz, G., Chisholm, C., Xu, X., Lahusen, T. J., Li, C., Sakamuru, Southward., et al. (2014). Drug repurposing screen identifies lestaurtinib amplifies the power of the poly (ADP-ribose) polymerase 1 inhibitor AG14361 to kill breast cancer associated factor-1 mutant and wild blazon breast cancer cells. Breast Cancer Res. 3, R67. doi:10.1186/bcr3682
PubMed Abstract | CrossRef Full Text | Google Scholar
Viel, T., Monfared, P., Schelhaas, S., Fricke, I. B., Kuhlmann, M. T., Fraefel, C., et al. (2013). Optimizing glioblastoma temozolomide chemotherapy employing lentiviral-based anti-MGMT shRNA technology. Mol. Ther. 3, 570–579. doi:x.1038/mt.2012.278
CrossRef Full Text | Google Scholar
Wang, B., Qiao, L., Wang, Y., Zeng, J., Chen, D., Guo, H., et al. (2018). Mitochondrial DNA D-loop lesions with the enhancement of DNA repair contribute to gastrointestinal cancer progression. Oncol. Rep. half dozen, 3694–3704. doi:10.3892/or.2018.6724
PubMed Abstruse | CrossRef Total Text | Google Scholar
Wang, Y., Xu, H., Liu, T., Huang, M., Butter, P. P., Li, C., et al. (2018). Temporal DNA-PK activation drives genomic instability and therapy resistance in glioma stem cells. JCI Insight. 3, e98096. doi:10.1172/jci.insight.98096
CrossRef Full Text | Google Scholar
Wang, Fifty. East., Yin, M., Dong, Q., Stewart, D. J., Merriman, K. W., Amos, C. I., et al. (2011). DNA repair chapters in peripheral lymphocytes predicts survival of patients with non-small-prison cell lung cancer treated with outset-line platinum-based chemotherapy. J. Clin. Oncol. 31, 4121–4128. doi:x.1200/JCO.2010.34.3616
PubMed Abstract | CrossRef Full Text | Google Scholar
Watson, Z. L., Yamamoto, T. M., McMellen, A., Kim, H., Hughes, C. J., Wheeler, L. J., et al. (2019). Histone methyltransferases EHMT1 and EHMT2 (GLP/G9A) maintain PARP inhibitor resistance in high-grade serous ovarian carcinoma. Clin. Epigenet. 1, 165. doi:10.1186/s13148-019-0758-two
CrossRef Full Text | Google Scholar
Wilson, R. H., Evans, T. J., Middleton, G. R., Molife, 50. R., Spicer, J., Dieras, 5., et al. (2017). A phase I study of intravenous and oral rucaparib in combination with chemotherapy in patients with advanced solid tumours. Br. J. Cancer. vii, 884–892. doi:10.1038/bjc.2017.36
PubMed Abstract | CrossRef Full Text | Google Scholar
Win, A. K., Immature, J. P., Lindor, N. K., Tucker, Chiliad. M., Ahnen, D. J., Young, Thousand. P., et al. (2012). Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort written report. J. Clin. Oncol. nine, 958–964. doi:10.1200/jco.2011.39.5590
PubMed Abstract | CrossRef Full Text | Google Scholar
Wise, H. C., Iyer, Thousand. V., Moore, Chiliad., Temkin, Due south. Chiliad., Gordon, S., Aghajanian, C., et al. (2019). Action of M3814, an oral Dna-PK inhibitor, in combination with topoisomerase II inhibitors in ovarian cancer models. Sci. Rep. 1, 18882. doi:10.1038/s41598-019-54796-6
PubMed Abstract | CrossRef Total Text | Google Scholar
Wu, X., Luo, Q., Zhao, P., Chang, Westward., Wang, Y., Shu, T., et al. (2019). MGMT-activated DUB3 stabilizes MCL1 and drives chemoresistance in ovarian cancer. Proc. Natl. Acad. Sci. U.s.a.A. 8, 2961–2966. doi:x.1073/pnas.1814742116
PubMed Abstract | CrossRef Full Text | Google Scholar
Yang, C., Wang, Q., Liu, X., Cheng, X., Jiang, X., Zhang, Y., et al. (2016). NU7441 enhances the radiosensitivity of liver cancer cells. Cell. Physiol. Biochem. 5, 1897–1905. doi:ten.1159/000445551
PubMed Abstract | CrossRef Total Text | Google Scholar
Yuzefovych, L. V., Kahn, A. G., Schuler, K. A., Eide, L., Arora, R., Wilson, G. L., et al. (2016). Mitochondrial DNA repair through OGG1 activity attenuates chest cancer progression and metastasis. Cancer Res. 1, 30–34. doi:ten.1158/0008-5472.tin can-15-0692
CrossRef Full Text | Google Scholar
Zhao, H., Wang, S., Song, C., Zha, Y., and Li, Fifty. (2016). The prognostic value of MGMT promoter status by pyrosequencing analysis for glioblastoma patients' survival: a meta-analysis. Globe J. Surg. Oncol. 1, 261. doi:10.1186/s12957-016-1012-4
PubMed Abstract | CrossRef Total Text | Google Scholar
Zhao, Q., Guan, J., Zhang, Z., Lv, J., Wang, Y., Liu, L., et al. (2017). Inhibition of Rad51 sensitizes breast cancer cells with wild-type PTEN to olaparib. Biomed. Pharmacother. 165–168. doi:10.1016/j.biopha.2017.07.090
CrossRef Full Text | Google Scholar
What Are Dna Repair Enzymes,
Source: https://www.frontiersin.org/articles/10.3389/fphar.2020.629266/full
Posted by: janssonsoublartand1963.blogspot.com


0 Response to "What Are Dna Repair Enzymes"
Post a Comment